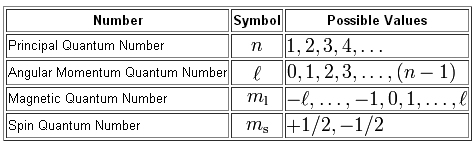
N1 N2 N3 N4 Chemistry

1 Hse I Part Iii Chemistry Max Marks 60 Max Time 2 Hrs Cool
Q Tbn 3aand9gcqalnpzwaqwgjd5n9bwfajqft1hm U8awiv6rbrjmzvsum6mkym Usqp Cau

Quantum Numbers And Electron Configurations
N 1 2 Hydroxy 3 Methoxybenzyl 4 Piperidinyl 2 Methoxy N 4 Methylphenyl Acetamide C23h30n2o4 Chemspider

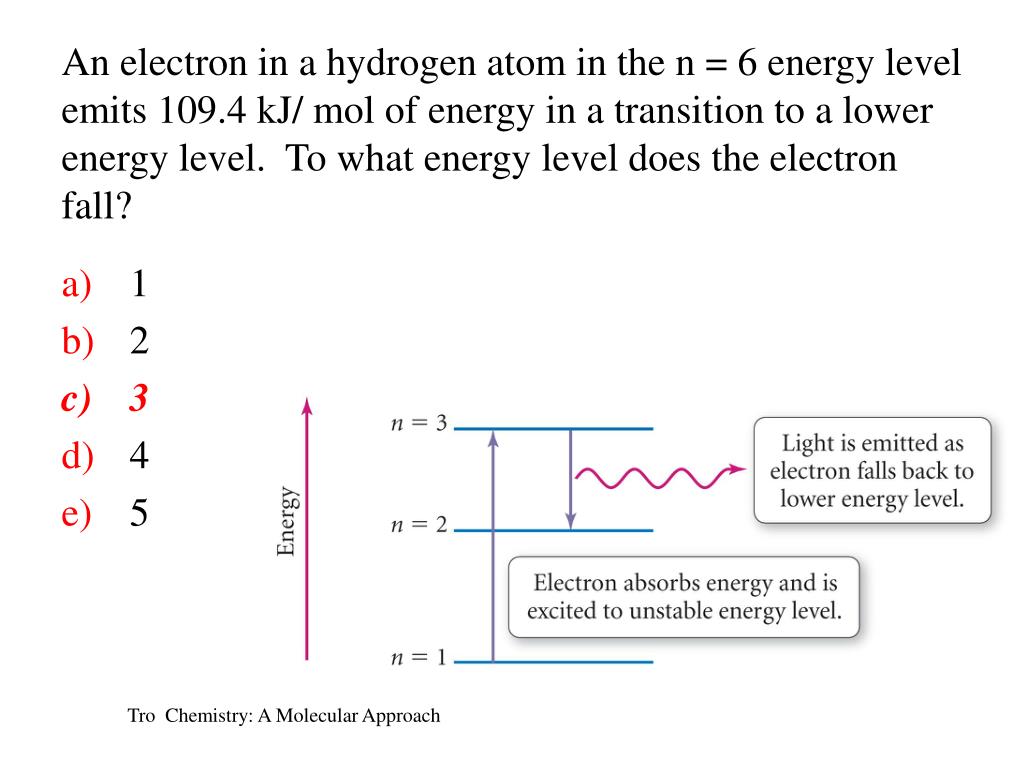
Determine Whether Each Of The Following Transitions In The H Clutch Prep
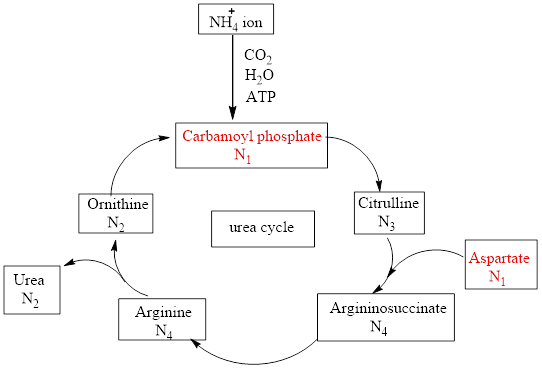
Characterize Each Of The Following Urea Cycle Compounds In Terms Of Its Nitrogen Content N 0 N 1 N 2 N 3 Or N 4 A Carbamoyl Phosphate B Ammonium Ion C Fumarate D Urea Bartleby
The orbital would be the same shape as the orbital but would be.
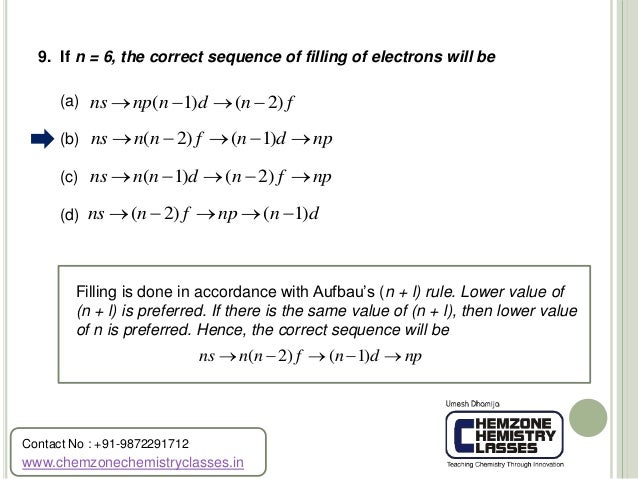
N1 n2 n3 n4 chemistry. 2~ "l" is the angular quantum number (l =n-1). 5 n = 5;. Which electronic transition in atomic hydrogen corresponds to the emission of visible light?a) n = 5 → n = 2b) n = 1 → n = 2c) n = 3 → n = 4d) n = 3 → n = 1 🤓 Based on our data, we think this question is relevant for Professor Waddell's class at UC.
N=infinity n=4 n=3 n=2 n=1 In the Bohr model of the hydrogen atom, the electron occupies distinct energy states. What is the property that state you can rewrite an equation?. Classify each of the following nitrogen-containing entities as an N 1 , N 2 , N 3 , or N 4 species.
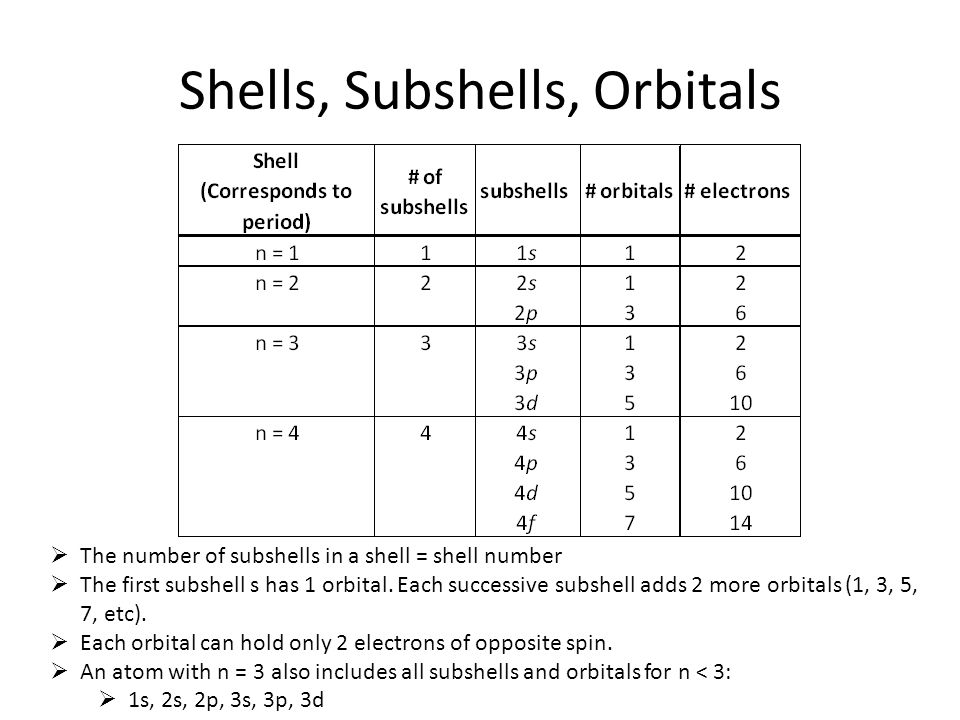
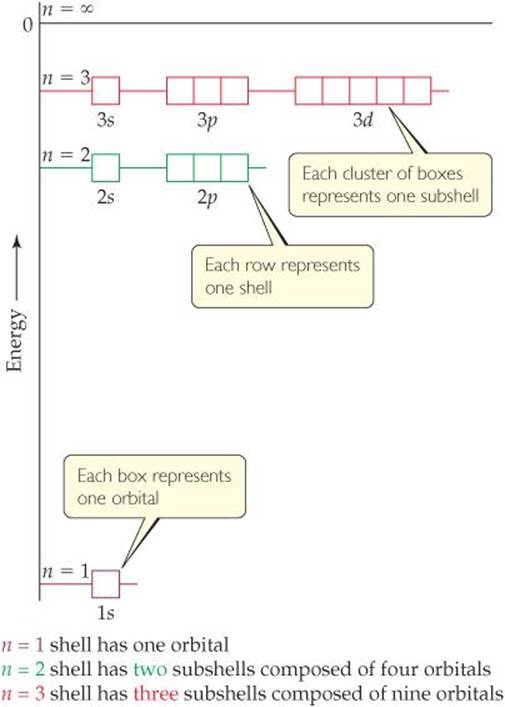
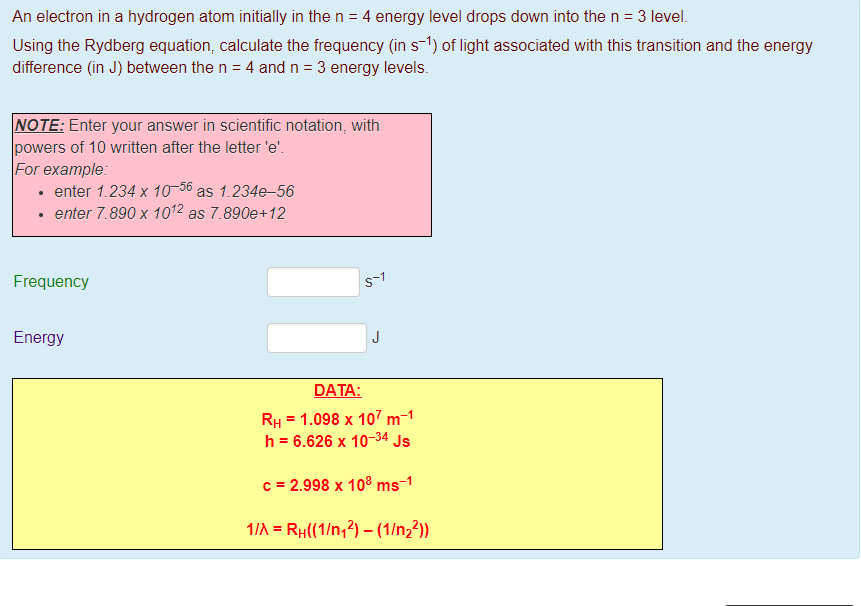
The n=3 shell contains 9 orbitals (max:. Factorial sign(!) is used to denote the product n.(n-1).(n-2).(n-3).(n-4)……….4.3.2.1. In the n=1 shell you only find s orbitals, in the n=2 shell, you have s and p orbitals, in the n=3 shell, you have s, p and d orbitals and in the n=4 up shells you find all four types of orbitals.
Are all part of an empirical theory designed to explain what we observe with respect to molecular structure and bonding. Get answers by asking now. $\begingroup$ Note, if you wanted to subvert the problem stated, you could perform induction separately on $\sum n^2$ and $\sum n$.
1^2 + 2^2 + 3^2 +. It is important to note here that these orbitals, shells etc. Join Yahoo Answers and get 100 points today.
Calculate maximum Number of e’s in n=4. Solve for n 1/(n-4)-2/n=3/(4-n) Find the LCD of the terms in the equation. Solution for -- = 00 n = 4 Cn = 3 II II В (i) n = 2 (ii) A -218- -n = 1 (iii) (b) Calculate the energy of the photon emitted for each transition.
3s, three 3p, and five 3d orbitals. If the wavelength of line B is 142.5 nm, calculate the wavelength of line A. Check all that apply.
For example, for (a), Tx (n) = 2x (n) + 3 Tx 2 (n) = 2x 2 (n) + 3 Since T(ax 1 (n) + bx2 (n) = 2ax 1 (n) + bx2 (n) + 3. On the basis of an asymmetric, conjugated, partially or wholly deprotonated 5-(pyridin-3-yl)-1H-pyrazole-3-carboxylic acid (H2ppc), five new complexes Co2(ppc)2(H2O. Simple and best practice solution for 3(n-4)=2(n-1) equation.
N=1, l=0, m=0 n=1, l=0, m=0 n=1, l=0, m=0 n = 2. Ask question + 100. X(n) = -26(n + 3) -6(n) + 36(n - 1) + 26(n - 3) Solution 2.
Find the sum of the first n terms of the series #1+2(1+1/n) +3(1+1/n)^2 + 4(1+1/n)^3# Using the agp ( arithmerico - geometrico progression ) sum formula?. This subproblem is being ignored because a solution could not be determined. Let a n = n2=(n4 + 1).
The characteristic equation is r3 -7r2 +16r-12 = 0. Firstly you failed to notice the pattern correctly since 2^n means 2^1+2^2+2^3 instead of what is shown. Learn with Tiger how to do 2/3(1+n)=-1/2n fractions in a clear and easy way :.
We know that jsinnj<1, so nsin2 n n3 + 1 n n3 + 1 n. Explain a formula Dear Dr. If observed closely, we can see that, if we take n common, series turns into an Harmonic Progression.
Log in with Facebook Log in with Google Log in with email Join using Facebook Join using Google. 5 p 3 s n = 4 4 d n = 3 There is a shorthand notation that can be used. Textbook solution for Organic And Biological Chemistry 7th Edition STOKER Chapter 15 Problem 15.76EP.
N = 1, n = 2, n = 3, n = 4, 1. 4 n = 4;. 1 + 2 + 3 +.
Page 2 of 7 8. The title coordination polymer, {Cd2(CH2N5)(C6H4NO2)Cl(OH)·0.14H2O}n, (I), was synthesized by the reaction of cadmium acetate and N-(1H-tetrazol-5-yl)isonicotinamide in aqueous ammonia, using hydrochloric acid to adjust the pH. The normality of 10% (weight/volume) acetic acid is (1) 1 N (2) 10 N (3) 1.7 N (4) 0. N ml of OEM UCL mived with ml of M.
09/14/02 at 10:33:49 From:. So its roots are 2, 2, and 3. Finding the LCD of a list of values is the same as finding the LCM of the denominators of those values.
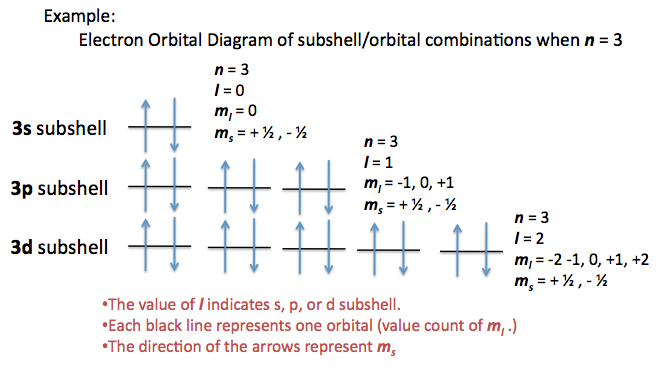
I mean, look at it for a second. Orbitals that have the same value of the principal quantum number form a shell.Orbitals within a shell are divided into subshells that have the same value of the angular quantum number. .
Finding n(n-1)/2 in the Real World Date:. Answer to Question 14 Look at the four sets of quantum numbers below. Tap for more steps.
Hence, we have e1/n n3/2 e n3/2 Since P en−3/2 converges (it’s a p-series with p = 3/2 > 1), the comparison test implies that P e1/nn−3/2 also converges. F(n-1) * F(n+1) = (-1)^n + (Fn)^2 I know I do a base case of n being 0, and then an inductive step of n being n+1, but beyond that I just can't get the math to work out. P 1 n=1 nsin2 3+1 Answer:.
+ n = (n(n+1))/2 for n, n is a natural number Step 1:. We have step-by-step solutions for your textbooks written by Bartleby experts!. N = 5.
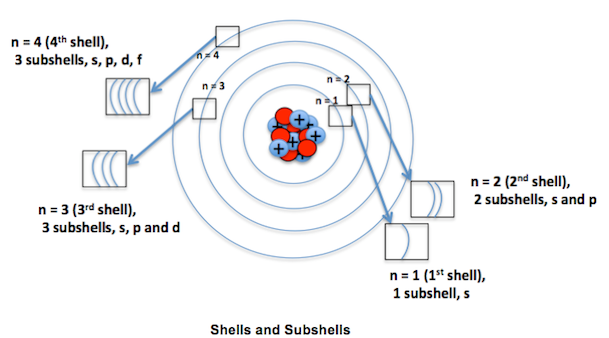
2 n = 2;. Get hold of all the important DSA concepts with the DSA Self Paced Course at a student-friendly price and become industry ready. It takes more energy to ionize (completely remove) the electron from n=3 than from the ground state b.
All atomic orbitals with n=10 are presented here.Note that the orbitals with negative m are identical to those with the same magnitude positive m value except for a rotation,and are not shown separately. Simplifying 24 + 50n + 35n 2 + 10n 3 + n 4 = 0 Solving 24 + 50n + 35n 2 + 10n 3 + n 4 = 0 The solution to this equation could not be determined. If an electron of hydrogen atom jumps from n=4 to n=2 energy state, which wavelength of light will be emitted?.
Find an answer to your question n(n+1)(n+2)(n+3)(n+4) Kylie borrowed a book from the library the library charge to fix rental for the book in late fee for every day the book was overdue expression below s …. N = 1 B. N-1 -16 a n-2 +12 a n-3 + n 4 n , a 0 = -2, a 1 = 0, a 2 = 5 The associated homogeneous equation is a n = 7 a n-1 -16 a n-2 +12 a n-3.
N = 2 C. The wavelength of light emitted if the electron drops from n=3 to n=2 will be shorter than the wavelength of light emitted if the electrons fall from. F(n) = n 4n ,so there is a.
P 1 n=1 n2 4+1 Answer:. Chemistry Sign up Log in Excel in math and science. N=2, l=0 and two electrons are present in s-subshell.
Total electrons = 2+6+10+14=32. It refers to refers to the orientation of the orbital around the nucleus. As the energy of the electron increases, so does the principal quantum number, e.g., n = 3 indicates the third principal shell, n = 4 indicates the fourth principal shell, and so on.
+ 2^n = 2^(n-1) This makes absolutely no sense. < nn, where n 2 is an integer. 3 n = 3;.
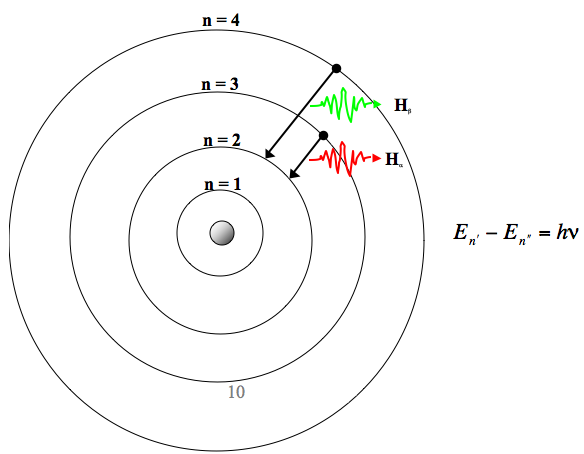
0ne transition between energy states of the hydrogen atom is represented by the picture on the left. It refers to refers to shape of the orbital (values of l allowed are zero and all positive integers less than or equal to n-1). Equivalent Fractions,Least Common Denominator, Reducing (Simplifying) Fractions Tiger Algebra Solver.
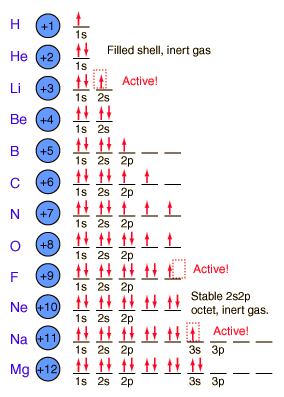
Set the factor '(24 + 50n + 35n 2 + 10n 3 + n 4)' equal to zero and attempt to solve:. Na is basically Ne 3 s 1 and Al is Ne 3 s 2 3 p 1. Related Answers At his donut shop, Jose is able to make 45 donuts in 30 minutes.
(1)College of Chemistry, Fuzhou University, Fuzhou , People's Republic of China. How would the 2s and 3p orbitals differ from the 1s and 2p orbitals?. Thus you don't have to sort the whole thing every time:.
How many orbitals in an atom can have the designation:. (the given statement)\ Let P(n):. Click here👆to get an answer to your question ️ (Dildly JUIULIUIT, CUlterill alivil Teris, SOIUDIIily) 1.
12/10/01 at 17:49:43 From:. How many sets are not allowed?. Since ex is a strictly increasing function, e1/n ≤ e for all n ≥ 1.
N/4+n/3=1/2 Find a common denominator for the L side of the equation. If f(n) = (n+2)(n+3) (n+1)3 then f′(n) = (2n+5)(n+1)3 − 3(n2 +5n+6)(n+1)2 (n+1)6 n2 +8n+13 (n +1)4. Shells and Subshells of Orbitals.
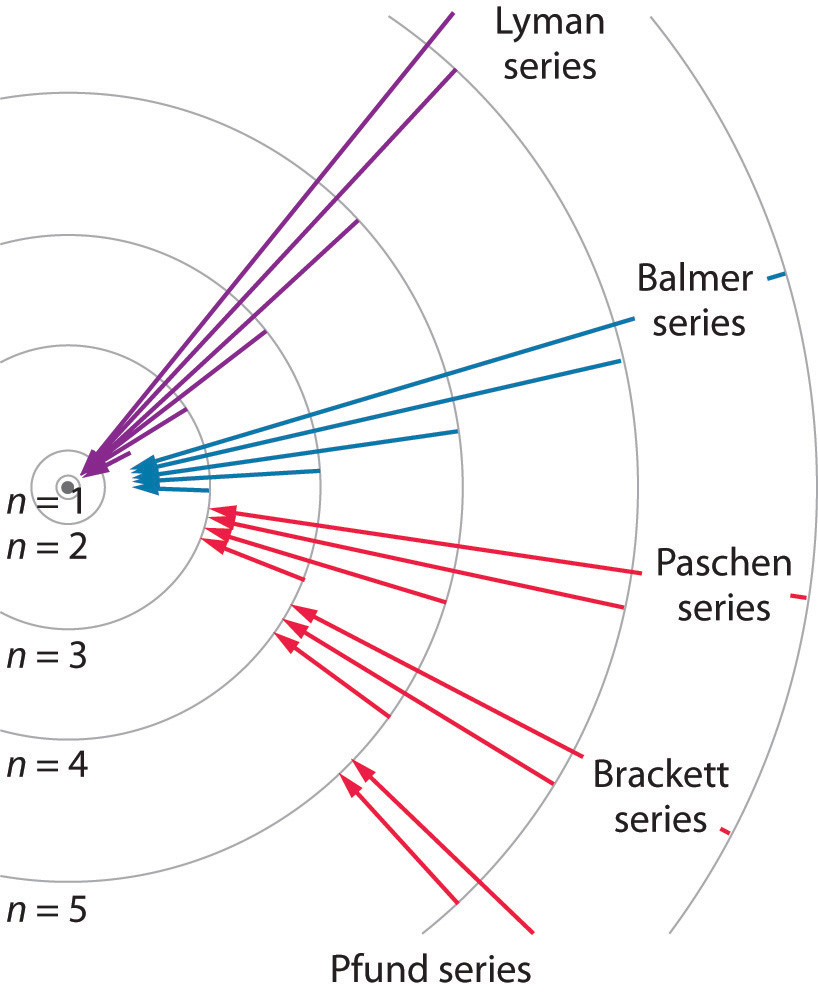
Lyman Series ( to n=1) n=2 to n=1:. You only need to sort n - 2 elements the second time through, n - 3 elements the third time, and so on. Chemistry , 01.11.19 14:31, adelarangelmartinez An atom of an element x has two electrons with n=1, eight electrons with n=2, eight electrons with n=3 and one electron with n=4.
The sum of (1+2^n)/3^n = 1/2 +2 =5/2. Free math problem solver answers your algebra, geometry, trigonometry, calculus, and statistics homework questions with step-by-step explanations, just like a math tutor. Our solution is simple, and easy to understand, so don`t hesitate to use it as a solution of your homework.
The n=2 shell of a carbon atom contains 2 electrons in the 2s orbital and 1 electron in each of two 2p orbitals (total number of electrons in the n=2 shell is 4 for carbon). Solve x^2 - 29x + 100=0?. Check how easy it is, and learn it for the future.
3n/12+4n/12=1/2 (3n+4n)/12=1/2 7n/12=1/2 7n/12*12=1/2*12 7n=6 7n*1/7=6*1/7 n=6/7 Substitute this value back into the original equation to make sure it is correct. For real-valued orbitals, as convenient in chemistry, look at Hydrogen orbitals 3D real. (We need to show that P(k + 1) is true, given the inductive hypothesis.).
B) n = 16 c) n = 8 d) n = 2 and the answer was a) n = 4. MAT V1102 – 004 Solutions:. Since n4 + 1 >n4, we have 1 n4+1 < 1 n4, so a n = n 2 n4 + 1 n n4 1 n2 therefore 0 <a n < 1 n2 Since the p-series P 1 n=1 1 2 converges, the comparison test tells us that the series P 1 n=1 n2 n4+1 converges also.
Math, How can n(n-1)/2 be explained in common everyday language?. $\endgroup$ – half-integer fan Feb 2 '13 at 13:. Don’t stop learning now.
If Jose speeds up his production by 5 minutes, how many donuts per minute will he be making?. < kk for some k 2. Use the smallest possible value for n.
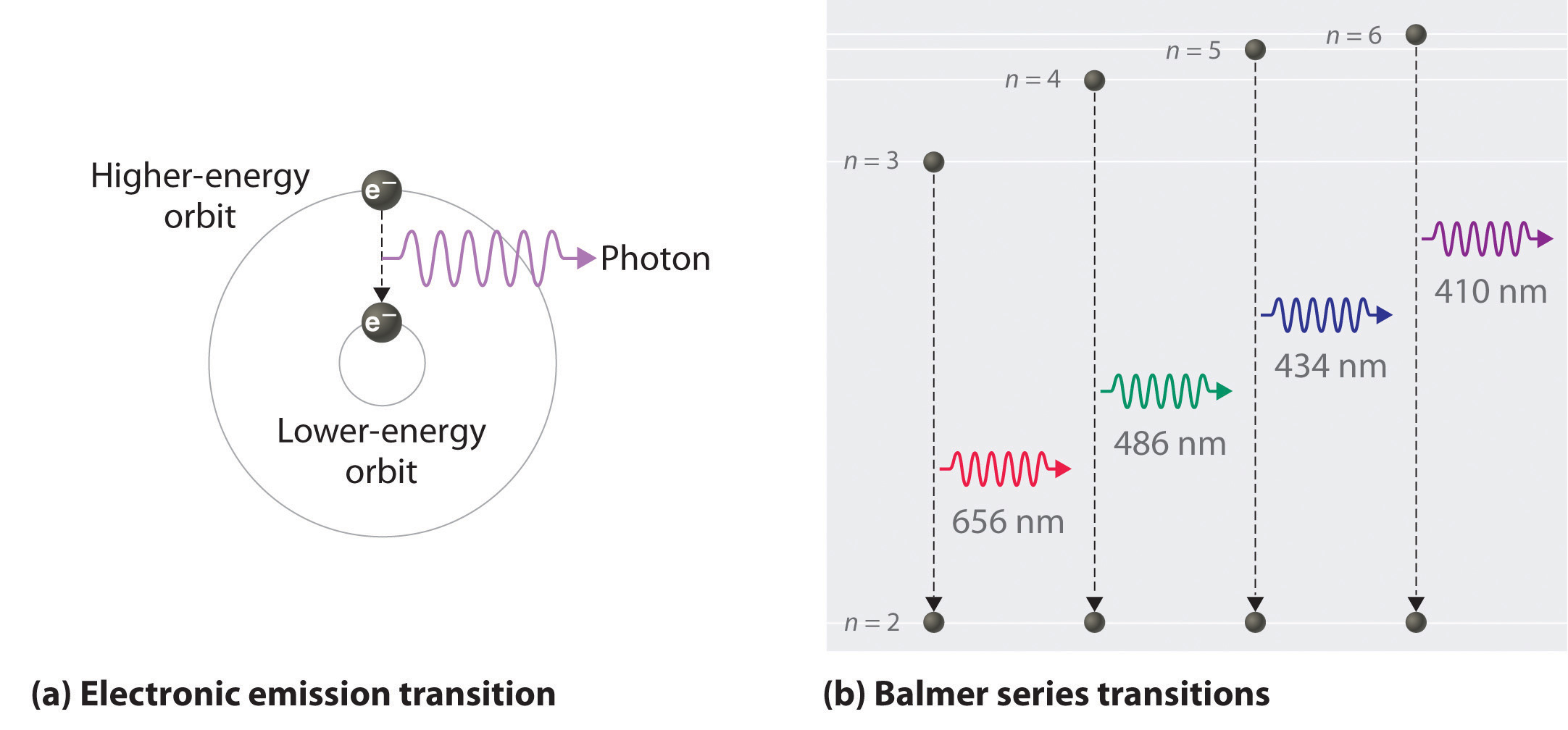
Known as emission, electrons can also "emit" energy as they jump to lower principle shells, where n decreases by whole numbers. 3~ "m" is the magnetic quantum number ( +/- l). Since n^3/(n^4-1)~n^3/n^4~1/n that is the harmonic series, that diverges.
+ n = (n(n+1))/2 Step. Question 12.What information does 2s 2 give about Q. Since contain both numbers and variables, there are four steps to find the LCM.
Form of Fibonacci numbers I am trying to create an inductive proof for the particular identity of Fibonacci numbers that:. 1 n = 1;. What is the wavelength of the transition from n=4 to n=3 for Li2+?.
Product of the integers 3. 4 s 3, n = n 4 + 6 n (n + 1) (2 n + 1) 6 − 4 n (n + 1) 2 + n s 3, n = 1 4 n 4 + 1 2 n 3 + 3 4 n 2 + 1 4 n − 1 2 n 2. Prove 1 + 2 + 3 +.
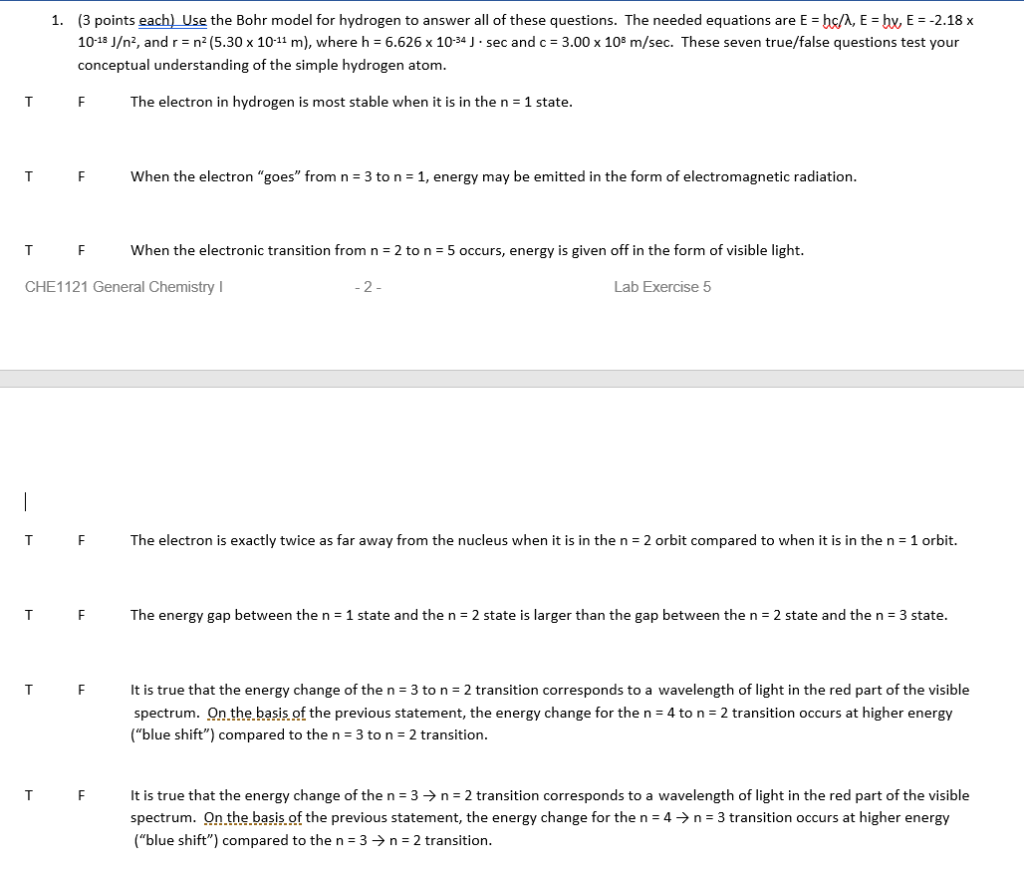
6 n = 6;. The electron is farther from the nucleus on average in the n=3 state then in the n=1 state c. CS240 Solutions to Induction Problems Fall 09 1.Let P(n) be the statement that n!.
From this information only, determine the following wherever possible , where it is not possible, explain why it is not so. What are the (1) momentum (2) wavelength and (3) energy of photon that is emitted when hydrogen atom undergoes transition from n=3 to n=1?. = 2 1 = 2 < 4 = 22 Inductive hypothesis:.
N = 3 D. Balmer Series (to n=2) n=3 to n=2:. Asked by Robin on October 21, 08;.
When is it used?. Line A is the transition of n=6 to n=3 Line B is the transition of n=5 to n=3. N = 4 E.
N=2, l=0, m=0 n=2, l=0, m=0 n=2, l=0, m=0 n=2, l=1, m=-1. This is an arithmetic series, and the equation for the total number of times is (n - 1)*n / 2. N = 1.
3 Each of the systems given can be tested against the definitions of linearity and time invariance. That means that the total number of compare/swaps you have to do is (n - 1) + (n - 2) +. What information does 3d 5 give about Q.
Chemists describe the shell and subshell in which an orbital belongs with a two-character code such as 2p or 4f.The first character indicates the shell (n = 2 or n = 4). N=3, l=2 and five electrons are present in d-subshell.

1 Electronic Structure Of Atoms Periodic Table Chapter 4 Chemistry Dacs 1232 Fakulti Kejuruteraan Mekanikal Utem Lecturer Imran Syakir Bin Mohamad Ppt Download
What Is The Difference Between A Shell And A Subshell Socratic
Solved 3 Points Each Use The Bohr Model For Hydrogen To Chegg Com
Question 300 Socratic
Quantum Mechanics And Atomic Orbitals Electronic Structure Of Atoms Chemistry The Central Science
6 3 Line Spectra And The Bohr Model Chemistry Libretexts

Quantum Numbers And Orbitals
Http Www Effinghamschools Com Cms Lib4 Ga Centricity Domain 364 Chapter 7 section 7 5 7 8 textbook homework answers Pdf

Quantum Number Wikipedia

N W Purin 6 Yl Aminoalkanoyl Derivatives Of Chiral Heterocyclic Amines As Promising Anti Herpesvirus Agents Krasnov 19 European Journal Of Organic Chemistry Wiley Online Library

Chapter 11 Modern Atomic Theory Chemistry 101 Structure Of Atom Rutherford S Model Source Of Particles E E Ppt Download
Orbitals

Energy Of Electron In The H Atom Is Determined By
Solved Please Fine The Answer For N1 N2 N3 N4 N5 N6 N7 N8 Chegg Com
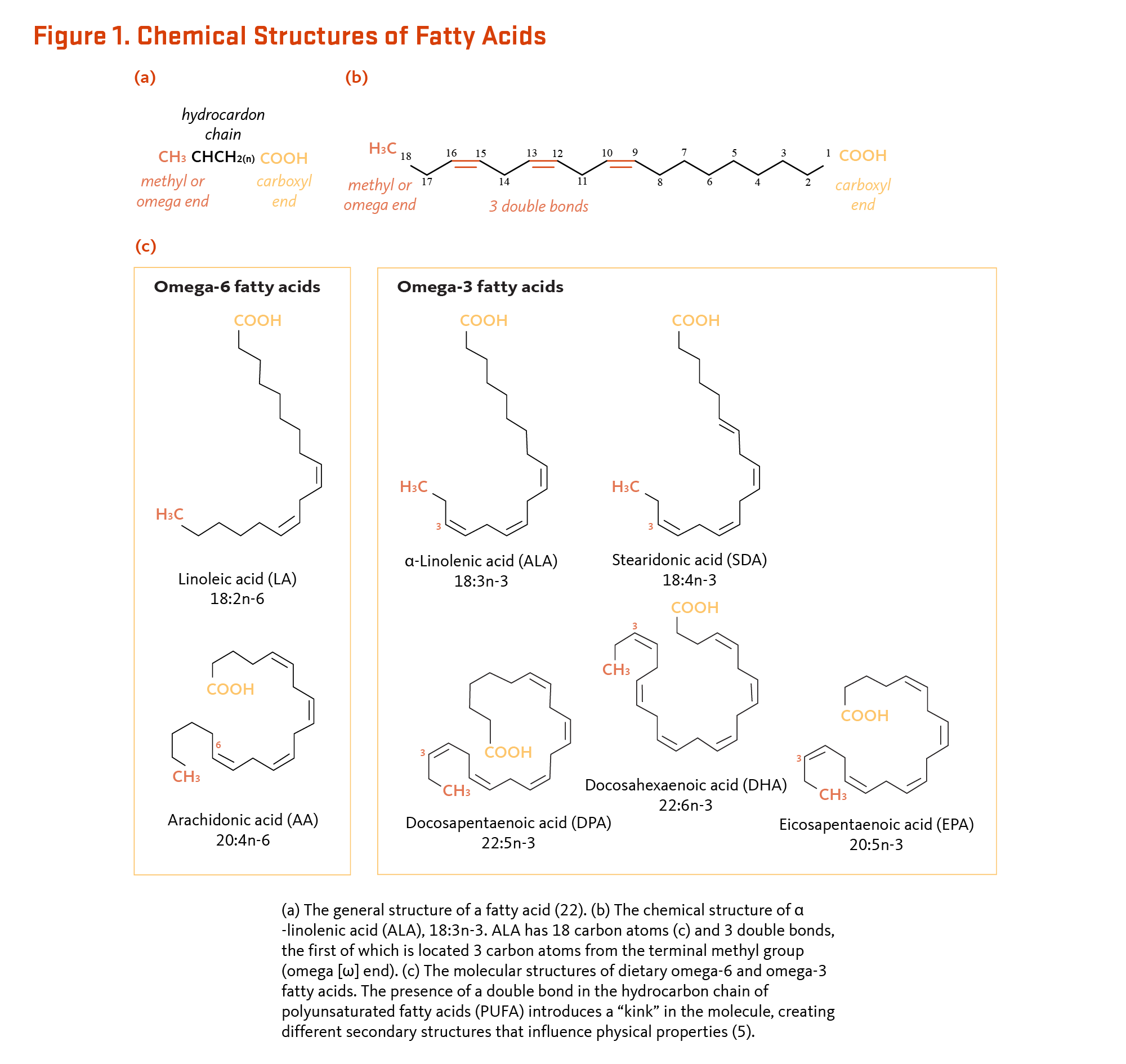
Essential Fatty Acids Linus Pauling Institute Oregon State University

Structure Of Atom Exercise With Solutions
Molecules Free Full Text Impact Of N Alkylamino Substituents On Serotonin Receptor 5 Htr Affinity And Phosphodiesterase 10a Pde10a Inhibition Of Isoindole 1 3 Dione Derivatives Html
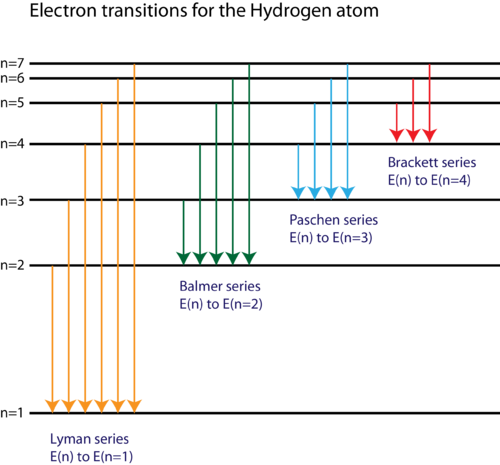
Spectral Lines Of Hydrogen Chemistry For Non Majors
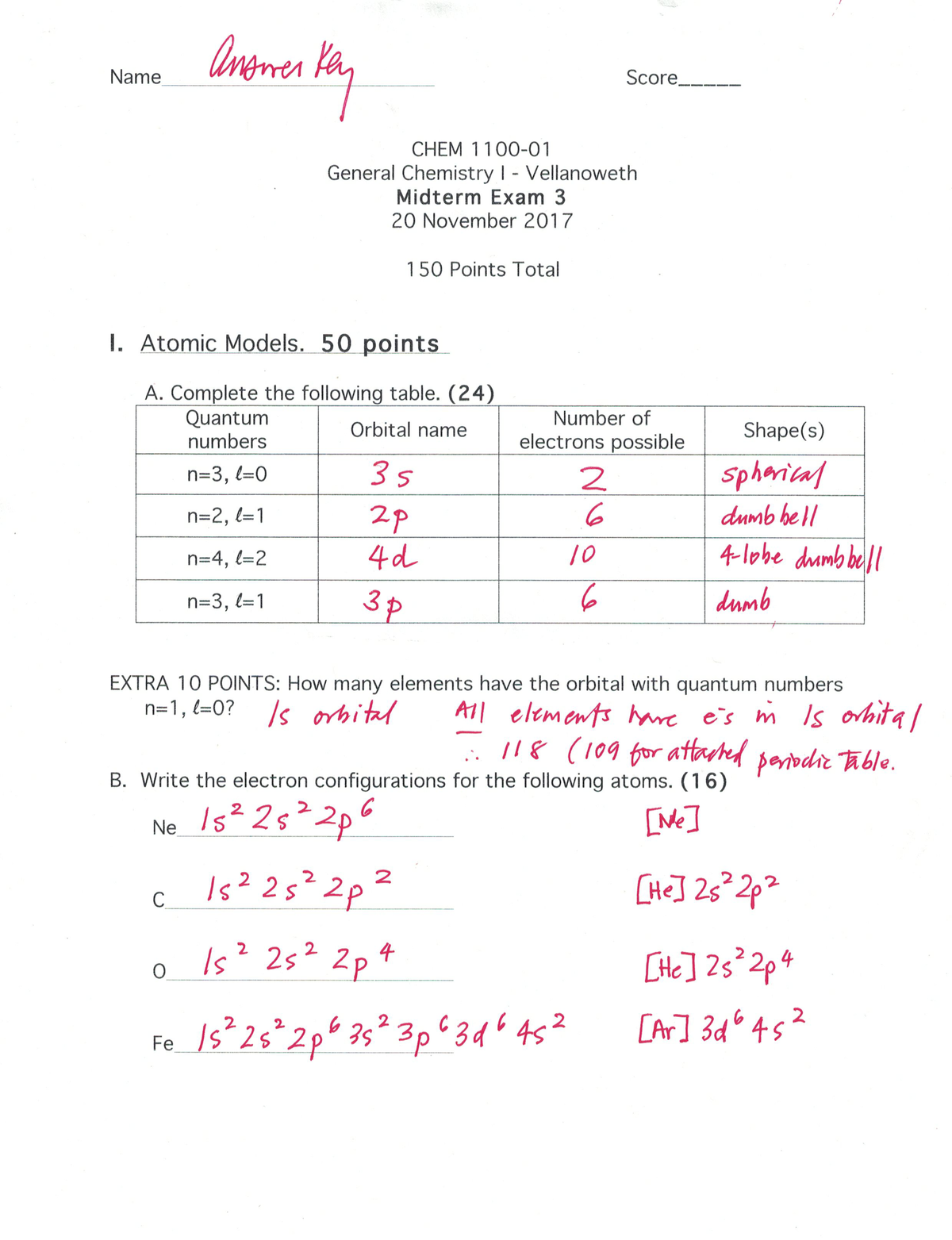
Exam Fall 17 Questions And Answers Chem 1100 Csula Studocu
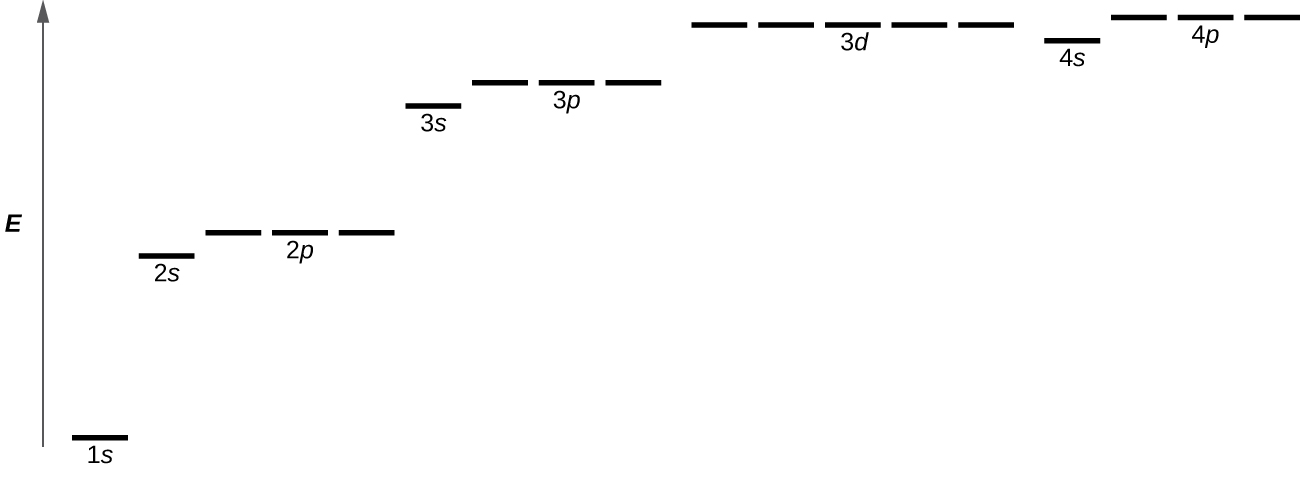
2 2 Atomic Orbitals And Quantum Numbers Chemistry Libretexts
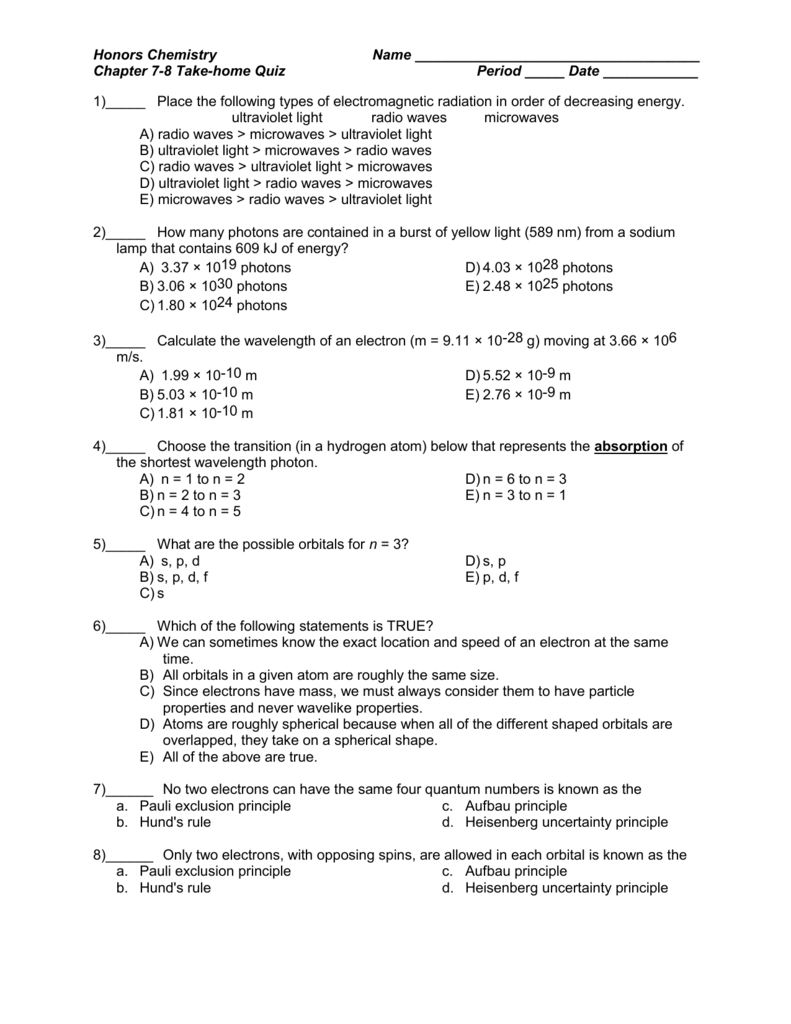
M T Thq Ch 7 8 Ehs

Honors Chemistry Worksheet Electronic Structure Of Atoms

Quantum Mechanics Orbitals General Chemistry Lecture 1140 Dr Sundin Uwp
2

Classnotes C14

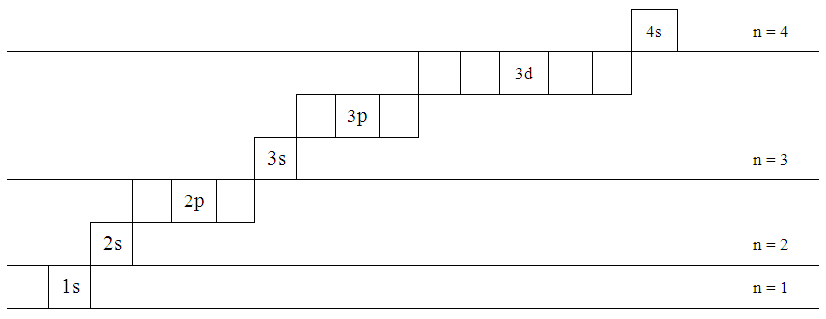
Diagnol Rule
Www Myhaikuclass Com Kristakyoung Chm080 Cms File Show Pdf T
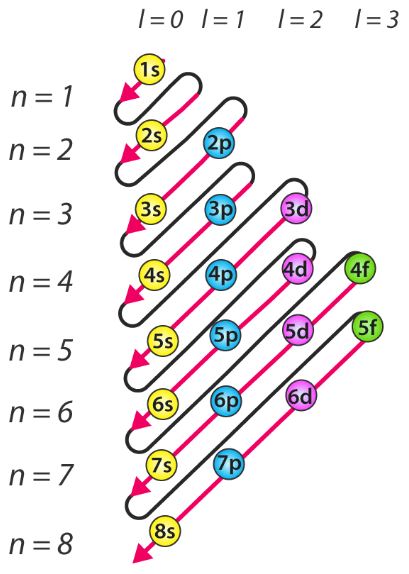
Electron Configurations Chemistry Community

Ap Chemistry

Neet Chemistry Mcq Atomic Structure 15 Youtube

Quantum Numbers And Electron Configurations

93 Best Chemistry Electron Configuration Images Chemistry Electron Configuration Teaching Chemistry

Bohr Emissions And Spectra Ppt Download

General Chemistry Questions Of Mcat Free Download

Quantum Numbers Video Quantum Physics Khan Academy

Chemistry The Central Science Chapter 6 Section 5

Crystal Structure Of Poly Bis M 2 Amino 4 5 Dicyanoimidazolato K2n1 N3 Trans Bis N N Dimethylformamide Ko Cadmium Topic Of Research Paper In Chemical Sciences Download Scholarly Article Pdf And Read For Free On Cyberleninka Open Science Hub

Chapter 10 Coordination Chemistry Ii Bonding Pages 1 22 Text Version Anyflip
Bohr S Atomic Model Line Spectrum Of H Atom Zeemann Stark Effect

Figure S4 Solid State Structure Of Complex 2 Hydrogen Atoms Were Download Scientific Diagram

Electronic Structure Of Atoms Chemistry Library Science Khan Academy

Chemistry Section 3 Chemical Bonding Chemical Compounds Flashcards Quizlet

Unit 4 Cp Chemistry Bohr Quantum Numbers Quantum Mechanical Model Ppt Download
Writing Electron Configuration An Algorithmic Approach

Chem 121 Lecture Notes Fall 16 Lecture 7 Rydberg Constant Uncertainty Principle Electronvolt

Quantum Numbers Atomic Orbitals And Electron Configurations
Quantum Numbers For Atoms Chemistry Libretexts
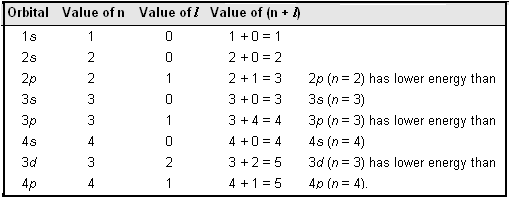
Subshell Shell N L Rule Value Of L May Be 0 1 2 3
2
2
Solved Hi I Have 2 Chemistry Questions This Already Has Chegg Com
Http Cdochemistrychristman Pbworks Com W File Fetch Unit 5 electronic structure and the periodic table problem set 5 2 answers 15 Pdf
Orbit Vs Orbitals In Chemistry Difference Explained With Diagram Viva Differences
Www Morehouse Edu Media Chemistry Brian Lawrence Genchem 111problems Ps08 Pdf

Atomic Structure Multiple Choice Questions For Iit Jee Neet Sat K
7 3 The Atomic Spectrum Of Hydrogen Chemistry Libretexts

1 Chemistry Of The Nonmetals

Design Synthesis And Biological Evaluation Of Bivalent Benzoxazolone And Benzothiazolone Ligands As Potential Anti Inflammatory Analgesic Agents Abstract Europe Pmc

Bulky Bis Aryl Triazenides Just Aspiring Amidinates A Structural And Spectroscopic Study Dalton Transactions Rsc Publishing
Numerical Characterization Of Chemical Fragments Molecules And Clusters Using Skeletal Numbers And Nuclearity Trees

A Molecule Absorbs Light Having A Specific Wavelength Why Doesn T It Absorb Shorter Wavelengths Chemistry Stack Exchange

Answered What Designations Are Given To The Bartleby
1
Quantum Numbers Introduction To Chemistry

Table 2 From Novel Bis Thiosemicarbazones Of The 3 5 Diacetyl 1 2 4 Triazol Series And Their Platinum Ii Complexes Chemistry Antiproliferative Activity And Preliminary Nephrotoxicity Studies Semantic Scholar

Coordination Chemistry Of An Unsymmetrical Naphthyridine Based Tetradentate Ligand Toward Various Transition Metal Ions Tsai 16 European Journal Of Inorganic Chemistry Wiley Online Library
Q Tbn 3aand9gcrlae0s7chaz6vpjdvtawuab8g5yehswqyjzjcwitn6n1r34lkw Usqp Cau

How Many Electrons In An Atom May Have The Following Quantum Number N 4 Ms N 3 Chemistry Structure Of Atom Meritnation Com
Ppt Chemistry A Molecular Approach 1 St Edition Nivaldo J Tro Powerpoint Presentation Id

Atomic Sub Shells

Click On A Subshell To See The Labels For All Orbitals In Th Clutch Prep
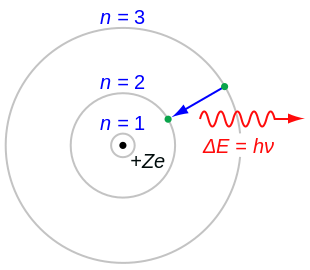
Bohr Model Wikipedia
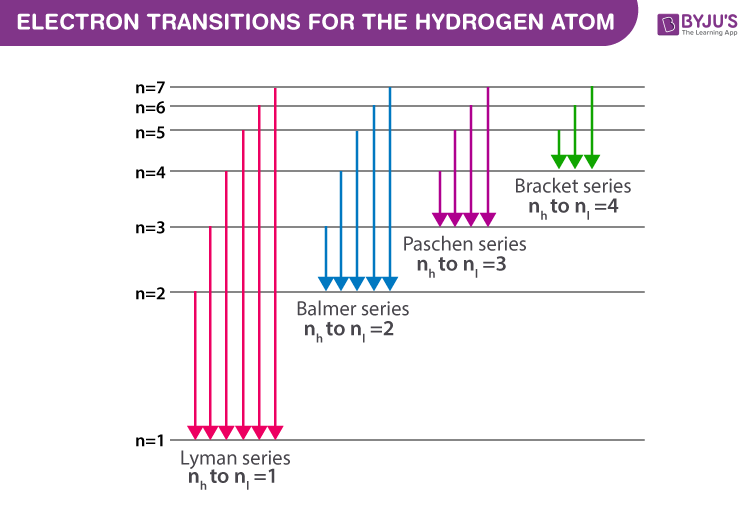
Spectral Series Explained Along With Hydrogen Spectrum Rydberg Formula

Aufbau Principle Wikipedia

Synthesis Biological And Antitumor Activity Of A Highly Potent 6 Substituted Pyrrolo 2 3 D Pyrimidine Thienoyl Antifolate Inhibitor With Proton Coupled Folate Transporter And Folate Receptor Selectivity Over The Reduced Folate Carrier That Inhibits B
Chemistry Dashboard Mixtures Components And Neutralized Forms Of N 2 N 2 N 4 N 6 Tetramethylmelamine Chemicals

The Quantum Mechanical Model Of The Atom Ppt Video Online Download

Quantum Numbers And Electron Configurations
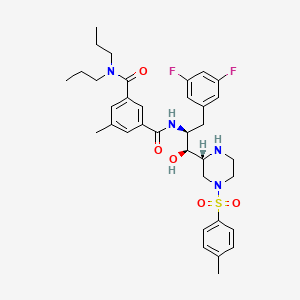
1 N 1s 2s 3 3 5 Difluorophenyl 1 Hydroxy 1 2r 4 4 Methylphenyl Sulfonylpiperazin 2 Yl Propan 2 Yl 5 Methyl 3 N 3 N Dipropylbenzene 1 3 Dicarboxamide C35h44f2n4o5s Pubchem

The Crystal Structure Of Tris Thiourea Copper I Perchlorate Cu Scn2h4 3clo4

Figure 4 From Polynitrogen Chemistry Synthesis Characterization And Crystal Structure Of Surprisingly Stable Fluoroantimonate Salts Of N5 Semantic Scholar

How Are Electrons Distributed In Different Orbits Electronic Configuration

اعداد کوانتومی آرایش الکترونی تناوب خواص Periodic Table Atomic Orbital

Homework 6 For Chemestry Chemistry I Che 1401 Studocu
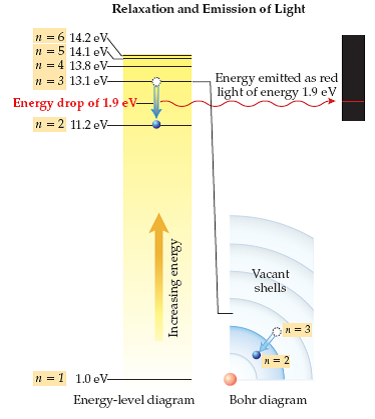
Solved Using The Energy Level Diagram Calculate The Wavelengt Chegg Com

Atomic Structure And Properties Sch4u Catherine Gordeyev
Www Studocu Com En Us Document Brigham Young University General College Chemistry Other Ps 08 19 Key Key View

Using S P D D Notation Describe The Orbitals With The Following Quantum Numbers 1 N 2 L 1 2 N 4 L 0 3 N 5 L 0 M 0 4 N 4 L 2 Chemistry Structure Of Atom Meritnation Com

The Sum Of The First N Terms Of An Arithmetic Sequence Video Lesson Transcript Study Com
Chemistry Dashboard Ms Ready Mappings Of Simazine Isotopes Pre Filtered Chemicals

Optimized Structures For The K 3 K 1 N 3 P Left And K 2 K 2 N 4 Download Scientific Diagram
Q Tbn 3aand9gcr5 Rzjlvcuo6 65ksuf48ebzkyuhh7eannyx6dxztqmoxx2btr Usqp Cau

Review Inorganic Chemistry Chemistry More Than Million Compounds Are Composed Of These 116 Elements Element Is A Substance Consists Of Identical Ppt Download
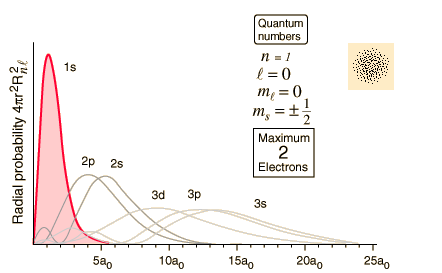
Orbitals

Quantum Numbers Atomic Orbitals And Electron Configurations

Chemistry The Bohr Model

Chemistry Electron Orbitals And Sub Levels
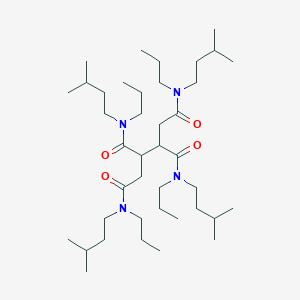
1 N 2 N 3 N 4 N Tetrakis 3 Methylbutyl 1 N 2 N 3 N 4 N Tetrapropylbutane 1 2 3 4 Tetracarboxamide C40h78n4o4 Pubchem



